Oxidation-Induced Photoluminescence of Conjugated Polymers
Ma. Helen M. Cativo, Amanda C. Kamps, Jian Gao, John K. Grey, Geoffrey R. Hutchison, So-Jung Park “Oxidation-Induced Photoluminescence of Conjugated Polymers” J. Phys. Chem. B (2013) 117(16), 4528-4535. DOI.
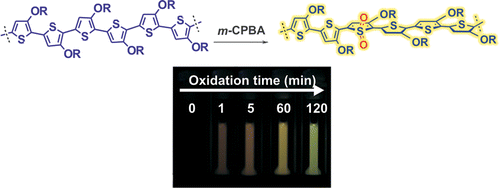 Here, we report an unusual oxidation-induced photoluminescence (PL) turn-on response of a poly(3-alkoxythiophene), poly(3-{2-[2-(2-ethoxyethoxy)ethoxy]ethoxy}thiophene) (PEEEET). PEEEET shows a significantly red-shifted absorption spectrum compared to polyalkylthiophenes and is almost nonfluorescent (quantum yield ≪ 1%) in its pristine state. The introduction of sulfonyl defects along the polymer backbone by the oxidation of PEEEET with meta-chloroperbenzoic acid (m-CPBA) increased the emission quantum yield with the intensity increasing with the degree of oxidation. Molecular modeling data indicated that the oxidation-induced PL increase cannot be explained by the nature of monomer units and radiative rate changes. We attributed the enhanced fluorescence to the reduced nonradiative rate caused by the increased band gap, according to the energy gap law, which is consistent with the observed blue shifts in absorption and PL spectra accompanied by the PL increase.
Here, we report an unusual oxidation-induced photoluminescence (PL) turn-on response of a poly(3-alkoxythiophene), poly(3-{2-[2-(2-ethoxyethoxy)ethoxy]ethoxy}thiophene) (PEEEET). PEEEET shows a significantly red-shifted absorption spectrum compared to polyalkylthiophenes and is almost nonfluorescent (quantum yield ≪ 1%) in its pristine state. The introduction of sulfonyl defects along the polymer backbone by the oxidation of PEEEET with meta-chloroperbenzoic acid (m-CPBA) increased the emission quantum yield with the intensity increasing with the degree of oxidation. Molecular modeling data indicated that the oxidation-induced PL increase cannot be explained by the nature of monomer units and radiative rate changes. We attributed the enhanced fluorescence to the reduced nonradiative rate caused by the increased band gap, according to the energy gap law, which is consistent with the observed blue shifts in absorption and PL spectra accompanied by the PL increase.