Piezoelectric effects of applied electric fields on hydrogen-bond interactions: first-principles electronic structure investigation of weak electrostatic interactions
Keith A. Werling, Geoffrey R. Hutchison, Daniel S. Lambrecht. “Piezoelectric effects of applied electric fields on hydrogen-bond interactions: first-principles electronic structure investigation of weak electrostatic interactions” J. Phys. Chem. Lett. (2013) 4(9) 1365-1370. Online
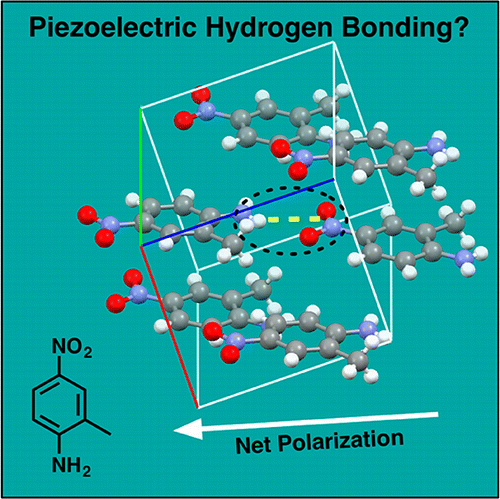 The piezoelectric properties of 2-methyl-4-nitroaniline crystals were explored qualitatively and quantitatively using an electrostatically embedded many-body (EE-MB) expansion scheme for the correlation energies of a system of monomers within the crystal. The results demonstrate that hydrogen bonding is an inherently piezoelectric interaction, deforming in response to the electrostatic environment. We obtain piezo-coefficients in excellent agreement with the experimental values. This approach reduces computational cost and reproduces the total resolution of the identity (RI)-Møller–Plesset second-order perturbation theory (RI-MP2) energy for the system to within 1.3 × 10–5%. Furthermore, the results suggest novel ways to self-assemble piezoelectric solids and suggest that accurate treatment of hydrogen bonds requires precise electrostatic evaluation. Considering the ubiquity of hydrogen bonds across chemistry, materials, and biology, a new electromechanical view of these interactions is required.
The piezoelectric properties of 2-methyl-4-nitroaniline crystals were explored qualitatively and quantitatively using an electrostatically embedded many-body (EE-MB) expansion scheme for the correlation energies of a system of monomers within the crystal. The results demonstrate that hydrogen bonding is an inherently piezoelectric interaction, deforming in response to the electrostatic environment. We obtain piezo-coefficients in excellent agreement with the experimental values. This approach reduces computational cost and reproduces the total resolution of the identity (RI)-Møller–Plesset second-order perturbation theory (RI-MP2) energy for the system to within 1.3 × 10–5%. Furthermore, the results suggest novel ways to self-assemble piezoelectric solids and suggest that accurate treatment of hydrogen bonds requires precise electrostatic evaluation. Considering the ubiquity of hydrogen bonds across chemistry, materials, and biology, a new electromechanical view of these interactions is required.