Piezoelectric hydrogen bonding: computational screening for a design rationale
Keith A. Werling, Maryanne Griffin, Geoffrey R. Hutchison, Daniel S. Lambrecht. “Piezoelectric hydrogen bonding: computational screening for a design rationale” J. Phys. Chem. A. (2014) 118(35) 1365-1370. Online
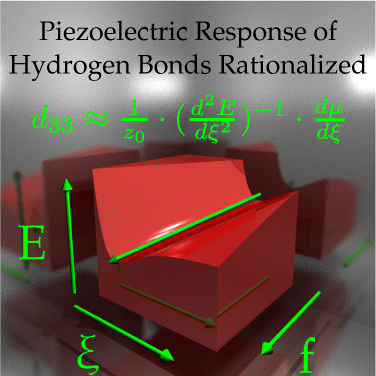 Organic piezoelectric materials are promising targets in applications such as energy harvesting or mechanical sensors and actuators. In a recent paper (Werling, K. A.; et al. J. Phys. Chem. Lett. 2013, 4, 1365–1370), we have shown that hydrogen bonding gives rise to a significant piezoelectric response. In this article, we aim to find organic hydrogen bonded systems with increased piezo-response by investigating different hydrogen bonding motifs and by tailoring the hydrogen bond strength via functionalization. The largest piezo-coefficient of 23 pm/V is found for the nitrobenzene–aniline dimer. We develop a simple, yet surprisingly accurate rationale to predict piezo-coefficients based on the zero-field compliance matrix and dipole derivatives. This rationale increases the speed of first-principles piezo-coefficient calculations by an order of magnitude. At the same time, it suggests how to understand and further increase the piezo-response. Our rationale also explains the remarkably large piezo-response of 150 pm/V and more for another class of systems, the “molecular springs” (Marvin, C.; et al. J. Phys. Chem. C 2013, 117, 16783–16790.).
Organic piezoelectric materials are promising targets in applications such as energy harvesting or mechanical sensors and actuators. In a recent paper (Werling, K. A.; et al. J. Phys. Chem. Lett. 2013, 4, 1365–1370), we have shown that hydrogen bonding gives rise to a significant piezoelectric response. In this article, we aim to find organic hydrogen bonded systems with increased piezo-response by investigating different hydrogen bonding motifs and by tailoring the hydrogen bond strength via functionalization. The largest piezo-coefficient of 23 pm/V is found for the nitrobenzene–aniline dimer. We develop a simple, yet surprisingly accurate rationale to predict piezo-coefficients based on the zero-field compliance matrix and dipole derivatives. This rationale increases the speed of first-principles piezo-coefficient calculations by an order of magnitude. At the same time, it suggests how to understand and further increase the piezo-response. Our rationale also explains the remarkably large piezo-response of 150 pm/V and more for another class of systems, the “molecular springs” (Marvin, C.; et al. J. Phys. Chem. C 2013, 117, 16783–16790.).