Sequence Effects in Conjugated Donor–Acceptor Trimers and Polymers
Shaopeng Zhang, Geoffrey R. Hutchison, and Tara Y. Meyer. “Sequence Effects in Conjugated Donor–Acceptor Trimers and Polymers” Macromol. Rapid Commun. (2016) 37(11) pp. 882–887. DOI
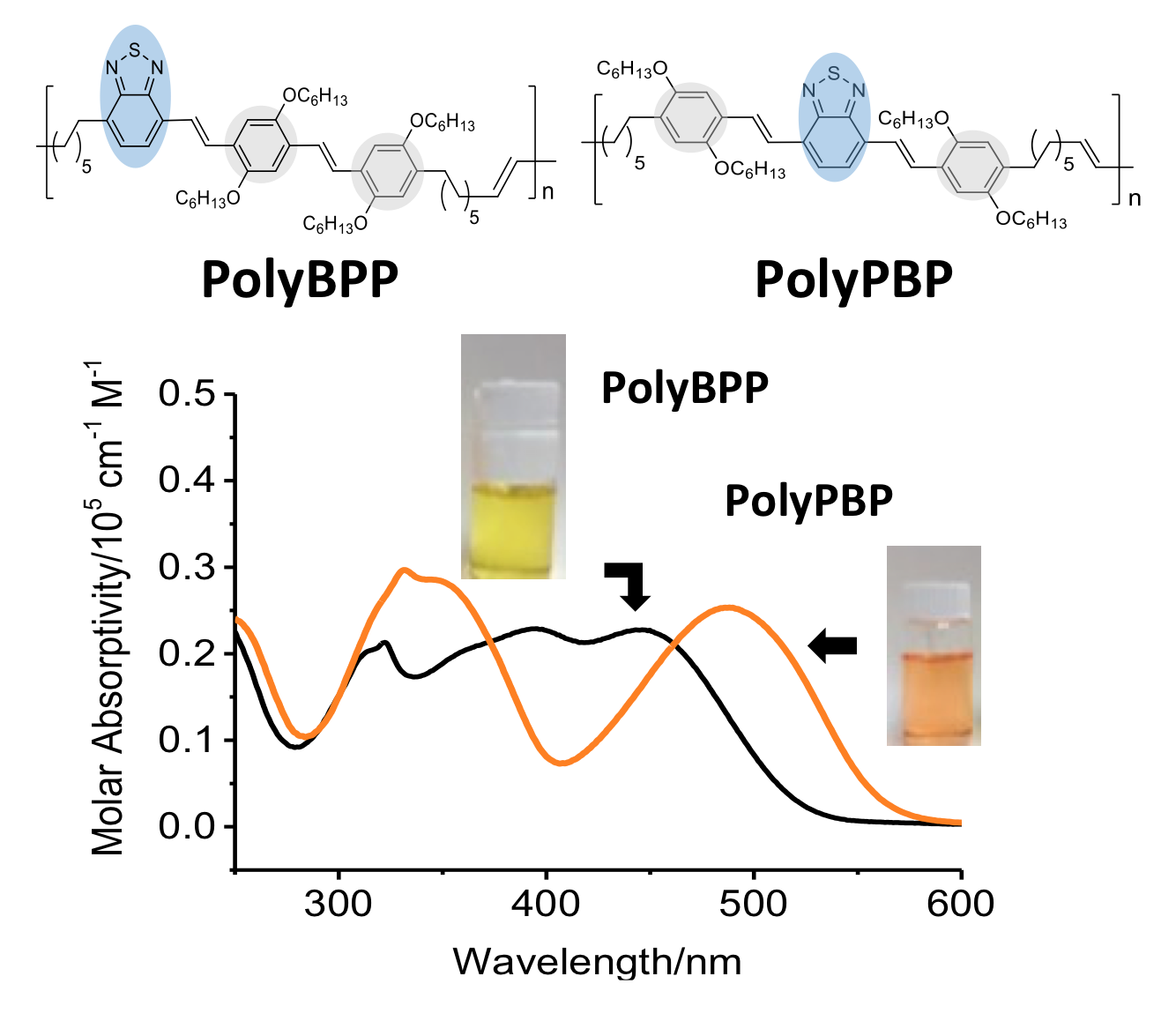 To investigate the sequence effect on donor–acceptor conjugated oligomers and polymers, the trimeric isomers PBP and BPP, comprising dialkoxy phenylene vinylene (P), benzothiadiazole vinylene (B), and alkyl endgroups with terminal olefins, are synthesized. Sequence effects are evident in the optical/electrochemical properties and thermal properties. Absorption maxima for PBP and BPP differ by 41 nm and the electrochemical band gaps by 0.1 V. The molar emission intensity is five times greater in PBP than BPP. Both trimers are crystalline and the melting points differ by 17 °C. The PBP and BPP trimers are used as macromonomers in an acyclic diene metathesis polymerization to give PolyPBP and PolyBPP. The optical and electrochemical properties are similar to those of their trimer precursors—sequence effects are still evident. These results suggest that sequence is a tunable variable for electronic materials and that the polymerization of oligomeric sequences is a useful approach to introducing sequence into polymers.
To investigate the sequence effect on donor–acceptor conjugated oligomers and polymers, the trimeric isomers PBP and BPP, comprising dialkoxy phenylene vinylene (P), benzothiadiazole vinylene (B), and alkyl endgroups with terminal olefins, are synthesized. Sequence effects are evident in the optical/electrochemical properties and thermal properties. Absorption maxima for PBP and BPP differ by 41 nm and the electrochemical band gaps by 0.1 V. The molar emission intensity is five times greater in PBP than BPP. Both trimers are crystalline and the melting points differ by 17 °C. The PBP and BPP trimers are used as macromonomers in an acyclic diene metathesis polymerization to give PolyPBP and PolyBPP. The optical and electrochemical properties are similar to those of their trimer precursors—sequence effects are still evident. These results suggest that sequence is a tunable variable for electronic materials and that the polymerization of oligomeric sequences is a useful approach to introducing sequence into polymers.