Interplay among Sequence, Folding Propensity, and Bio-Piezoelectric Response in Short Peptides and Peptoids
Christopher W Marvin, Haley M Grimm, Nathaniel C Miller, W Seth Horne, Geoffrey R Hutchison. “Interplay among Sequence, Folding Propensity, and Bio-Piezoelectric Response in Short Peptides and Peptoids” J. Phys. Chem. B (2017) 121(44) 10269-10275. DOI
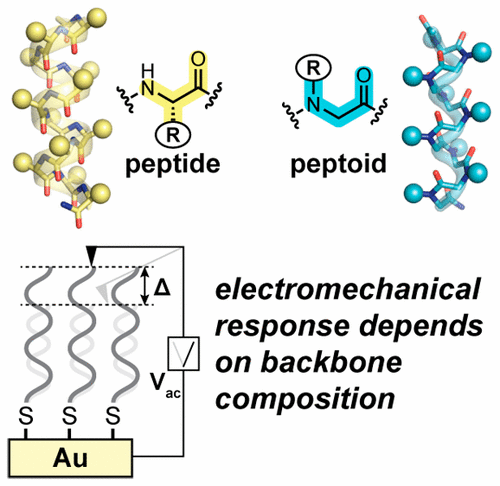 Many biomaterials are piezoelectric (i.e., mechanically deform under an applied electric field); however, the molecular origin of this phenomenon remains unclear. In the case of protein-based scaffolds, one possibility involves flexible response of local folding motifs to the applied field. Here, we test this hypothesis by examining the piezoresponse in a series of helical peptide-based oligomers. Control over folding propensity is exerted through systematic variation in both side-chain sequence and backbone composition. Piezoresponse is quantified by piezo-force microscopy on polar self-assembled monolayers. The results indicate backbone rigidity is an important determinant in peptide electromechanical responsiveness.
Many biomaterials are piezoelectric (i.e., mechanically deform under an applied electric field); however, the molecular origin of this phenomenon remains unclear. In the case of protein-based scaffolds, one possibility involves flexible response of local folding motifs to the applied field. Here, we test this hypothesis by examining the piezoresponse in a series of helical peptide-based oligomers. Control over folding propensity is exerted through systematic variation in both side-chain sequence and backbone composition. Piezoresponse is quantified by piezo-force microscopy on polar self-assembled monolayers. The results indicate backbone rigidity is an important determinant in peptide electromechanical responsiveness.